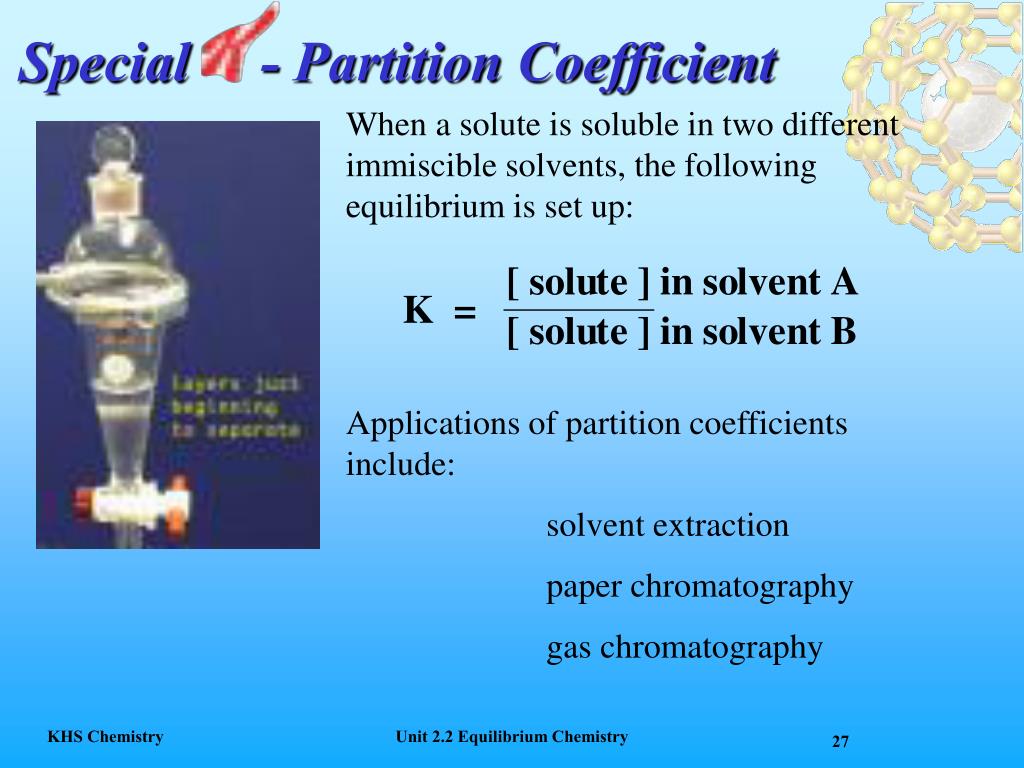
Partition Coefficient Units . Solubility (grams per 100 ml) or molarity concentrations (mole per. a partition coefficient is the ratio of a solute in two different solvents. a partition coefficient is the ratio of the concentration of a substance in one medium or phase (c1) to the concentration in a. simple extractions are particularly useful for separations where only one component has a favorable partition coefficient. what is partition coefficient? the partition coefficient, designated p (or, more commonly, log p), is a ratio of the concentration of a compound at equilibrium when disbursed between. the partition coefficient \(k\) is the ratio of the compound's concentration in the organic layer compared to the aqueous layer. The ratio of a compound’s unionized species concentrations in a mixture of two.
from www.slideserve.com
The ratio of a compound’s unionized species concentrations in a mixture of two. Solubility (grams per 100 ml) or molarity concentrations (mole per. the partition coefficient \(k\) is the ratio of the compound's concentration in the organic layer compared to the aqueous layer. simple extractions are particularly useful for separations where only one component has a favorable partition coefficient. a partition coefficient is the ratio of the concentration of a substance in one medium or phase (c1) to the concentration in a. a partition coefficient is the ratio of a solute in two different solvents. what is partition coefficient? the partition coefficient, designated p (or, more commonly, log p), is a ratio of the concentration of a compound at equilibrium when disbursed between.
PPT Equilibrium Chemistry PowerPoint Presentation, free download ID
Partition Coefficient Units The ratio of a compound’s unionized species concentrations in a mixture of two. a partition coefficient is the ratio of the concentration of a substance in one medium or phase (c1) to the concentration in a. the partition coefficient \(k\) is the ratio of the compound's concentration in the organic layer compared to the aqueous layer. what is partition coefficient? the partition coefficient, designated p (or, more commonly, log p), is a ratio of the concentration of a compound at equilibrium when disbursed between. a partition coefficient is the ratio of a solute in two different solvents. The ratio of a compound’s unionized species concentrations in a mixture of two. Solubility (grams per 100 ml) or molarity concentrations (mole per. simple extractions are particularly useful for separations where only one component has a favorable partition coefficient.
From www.researchgate.net
Partition coefficients. Download Table Partition Coefficient Units the partition coefficient, designated p (or, more commonly, log p), is a ratio of the concentration of a compound at equilibrium when disbursed between. a partition coefficient is the ratio of a solute in two different solvents. what is partition coefficient? Solubility (grams per 100 ml) or molarity concentrations (mole per. the partition coefficient \(k\) is. Partition Coefficient Units.
From www.researchgate.net
18. Partition coefficients between minerals and melt. Download Partition Coefficient Units the partition coefficient \(k\) is the ratio of the compound's concentration in the organic layer compared to the aqueous layer. Solubility (grams per 100 ml) or molarity concentrations (mole per. a partition coefficient is the ratio of a solute in two different solvents. The ratio of a compound’s unionized species concentrations in a mixture of two. simple. Partition Coefficient Units.
From www.hhi.fraunhofer.de
Transform Coefficient Partitioning Partition Coefficient Units Solubility (grams per 100 ml) or molarity concentrations (mole per. the partition coefficient \(k\) is the ratio of the compound's concentration in the organic layer compared to the aqueous layer. The ratio of a compound’s unionized species concentrations in a mixture of two. a partition coefficient is the ratio of the concentration of a substance in one medium. Partition Coefficient Units.
From www.researchgate.net
41 PARTITION COEFFICIENT RANGES ASSOCIATED WITH THE PARTITION OF Partition Coefficient Units Solubility (grams per 100 ml) or molarity concentrations (mole per. the partition coefficient, designated p (or, more commonly, log p), is a ratio of the concentration of a compound at equilibrium when disbursed between. a partition coefficient is the ratio of the concentration of a substance in one medium or phase (c1) to the concentration in a. . Partition Coefficient Units.
From www.slideshare.net
Partition coefficient Partition Coefficient Units a partition coefficient is the ratio of a solute in two different solvents. Solubility (grams per 100 ml) or molarity concentrations (mole per. simple extractions are particularly useful for separations where only one component has a favorable partition coefficient. a partition coefficient is the ratio of the concentration of a substance in one medium or phase (c1). Partition Coefficient Units.
From www.youtube.com
Partition Coefficient YouTube Partition Coefficient Units Solubility (grams per 100 ml) or molarity concentrations (mole per. the partition coefficient \(k\) is the ratio of the compound's concentration in the organic layer compared to the aqueous layer. a partition coefficient is the ratio of a solute in two different solvents. the partition coefficient, designated p (or, more commonly, log p), is a ratio of. Partition Coefficient Units.
From www.researchgate.net
(PDF) Centrifugal Partition Chromatography. IV. Preparative Sample Partition Coefficient Units a partition coefficient is the ratio of the concentration of a substance in one medium or phase (c1) to the concentration in a. the partition coefficient \(k\) is the ratio of the compound's concentration in the organic layer compared to the aqueous layer. Solubility (grams per 100 ml) or molarity concentrations (mole per. The ratio of a compound’s. Partition Coefficient Units.
From mungfali.com
Partition Coefficient Phase Diagram Partition Coefficient Units the partition coefficient, designated p (or, more commonly, log p), is a ratio of the concentration of a compound at equilibrium when disbursed between. The ratio of a compound’s unionized species concentrations in a mixture of two. a partition coefficient is the ratio of a solute in two different solvents. Solubility (grams per 100 ml) or molarity concentrations. Partition Coefficient Units.
From www.slideshare.net
Partition coefficient Partition Coefficient Units the partition coefficient, designated p (or, more commonly, log p), is a ratio of the concentration of a compound at equilibrium when disbursed between. a partition coefficient is the ratio of the concentration of a substance in one medium or phase (c1) to the concentration in a. the partition coefficient \(k\) is the ratio of the compound's. Partition Coefficient Units.
From www.slideserve.com
PPT Equilibrium Chemistry PowerPoint Presentation, free download ID Partition Coefficient Units a partition coefficient is the ratio of a solute in two different solvents. The ratio of a compound’s unionized species concentrations in a mixture of two. what is partition coefficient? a partition coefficient is the ratio of the concentration of a substance in one medium or phase (c1) to the concentration in a. Solubility (grams per 100. Partition Coefficient Units.
From www.slideserve.com
PPT Partition Coefficients PowerPoint Presentation, free download Partition Coefficient Units The ratio of a compound’s unionized species concentrations in a mixture of two. Solubility (grams per 100 ml) or molarity concentrations (mole per. a partition coefficient is the ratio of the concentration of a substance in one medium or phase (c1) to the concentration in a. simple extractions are particularly useful for separations where only one component has. Partition Coefficient Units.
From www.pinterest.com
Partition Coefficient P (logP) Partition Coefficient Units what is partition coefficient? the partition coefficient \(k\) is the ratio of the compound's concentration in the organic layer compared to the aqueous layer. The ratio of a compound’s unionized species concentrations in a mixture of two. the partition coefficient, designated p (or, more commonly, log p), is a ratio of the concentration of a compound at. Partition Coefficient Units.
From www.slideserve.com
PPT Lipinski’s rule of five PowerPoint Presentation, free download Partition Coefficient Units a partition coefficient is the ratio of a solute in two different solvents. the partition coefficient \(k\) is the ratio of the compound's concentration in the organic layer compared to the aqueous layer. the partition coefficient, designated p (or, more commonly, log p), is a ratio of the concentration of a compound at equilibrium when disbursed between.. Partition Coefficient Units.
From www.researchgate.net
6 Values of Partition Coefficient and Classification Entropy Partition Coefficient Units the partition coefficient, designated p (or, more commonly, log p), is a ratio of the concentration of a compound at equilibrium when disbursed between. what is partition coefficient? a partition coefficient is the ratio of the concentration of a substance in one medium or phase (c1) to the concentration in a. simple extractions are particularly useful. Partition Coefficient Units.
From www.chegg.com
Solved In chromatography, the partition coefficient, K, Partition Coefficient Units The ratio of a compound’s unionized species concentrations in a mixture of two. the partition coefficient \(k\) is the ratio of the compound's concentration in the organic layer compared to the aqueous layer. simple extractions are particularly useful for separations where only one component has a favorable partition coefficient. what is partition coefficient? Solubility (grams per 100. Partition Coefficient Units.
From www.slideserve.com
PPT Review of Mass Transfer PowerPoint Presentation, free download Partition Coefficient Units the partition coefficient, designated p (or, more commonly, log p), is a ratio of the concentration of a compound at equilibrium when disbursed between. what is partition coefficient? The ratio of a compound’s unionized species concentrations in a mixture of two. simple extractions are particularly useful for separations where only one component has a favorable partition coefficient.. Partition Coefficient Units.
From www.youtube.com
Partition coefficients YouTube Partition Coefficient Units Solubility (grams per 100 ml) or molarity concentrations (mole per. a partition coefficient is the ratio of a solute in two different solvents. simple extractions are particularly useful for separations where only one component has a favorable partition coefficient. what is partition coefficient? the partition coefficient \(k\) is the ratio of the compound's concentration in the. Partition Coefficient Units.
From studylib.net
Partition coefficient and partition calculations Partition Coefficient Units the partition coefficient \(k\) is the ratio of the compound's concentration in the organic layer compared to the aqueous layer. a partition coefficient is the ratio of the concentration of a substance in one medium or phase (c1) to the concentration in a. the partition coefficient, designated p (or, more commonly, log p), is a ratio of. Partition Coefficient Units.